An automated method to find reaction mechanisms and solve the kinetics in organometallic catalysis
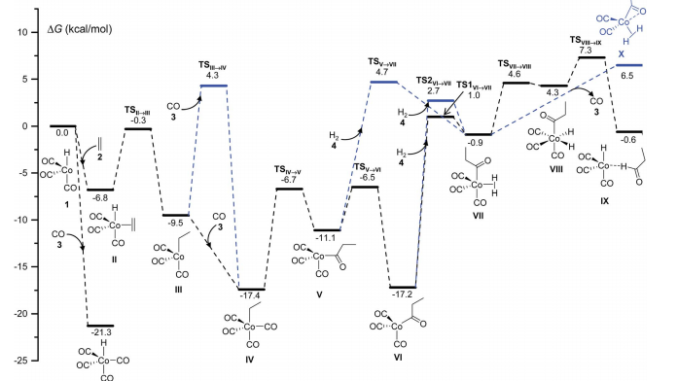
Abstract: A novel computational method is proposed in this work for use in discovering reaction mechanisms and solving the kinetics of transition metal-catalyzed reactions. The method does not rely on either chemical intuition or assumed a priori mechanisms, and it works in a fully automated fashion. Its core is a procedure, recently developed by one of the authors, that combines accelerated direct dynamics with an efficient geometry-based post-processing algorithm to find transition states (Martinez-Nunez, E., J. Comput. Chem. 2015, 36, 222-234). In the present work, several auxiliary tools have been added to deal with the specific features of transition metal catalytic reactions. As a test case, we chose the cobalt-catalyzed hydroformylation of ethylene because of its well-established mechanism, and the fact that it has already been used in previous automated computational studies. Besides the generally accepted mechanism of Heck and Breslow, several side reactions, such as hydrogenation of the alkene, emerged from our calculations. Additionally, the calculated rate law for the hydroformylation reaction agrees reasonably well with those obtained in previous experimental and theoretical studies.
Author(s): Varela, JA; Vazquez, SA; Martinez-Nunez, E
CHEMICAL SCIENCE
Volume: 8 Pages: 3843-3851 Published: MAY 1 2017
PDF: An automated method to find reaction mechanisms and solve the kinetics in organometallic catalysis
DOI: 10.1039/c7sc00549k