Writers: Rodrigo Marques FerreiraI, Maycon MottaI, Augusto Batagin-Neto, Carlos Frederico de Oliveira GraeffIII, Paulo Noronha Lisboa-Filho, Francisco Carlos Lavarda
Keywords: advanced electronic ceramics; density functional theory; infrared spectra simulations; Raman spectra simulations; sol-gel; barium complex
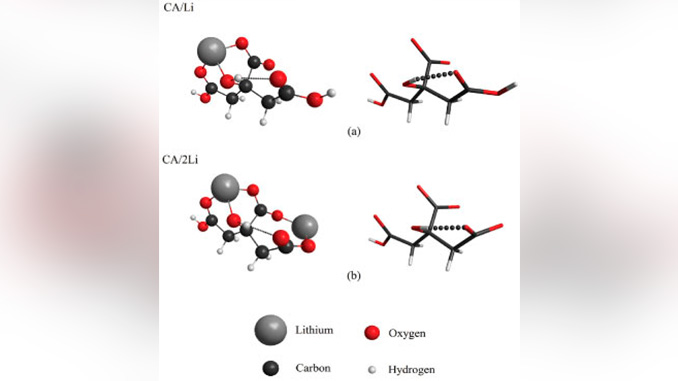
Abstract: The performance of advanced electronic ceramics is directly related to the synthesis route employed. Sol-gel methods are widely used for this purpose. However, the physicochemical intermediate steps are still not well understood. Better understanding and control of these processes can improve the final quality of samples. In this work, we studied theoretically the formation of metal complexes between citric acid and lithium or barium metal cations with different citric acid/metal proportions, using Density Functional Theory electronic structure calculations. Infrared and Raman scattering spectra were simulated for the more stable geometric configurations. Using this methodology, we identified some features of complexes formed in the synthesis process. Our results show that the complexes can be distinguished by changes in the bands assigned to C=O, COH-, and COO– group vibrations. An estimate of the most stable complexes is made based on total energy.