Writers: P. Francatto and F.N. Souza Neto and A.E. Nogueira and A.M. Kubo and L.S. Ribeiro and L.P. Gonçalves and L.F. Gorup and E.R. Leite and E.R. Camargo
Keywords: Nanopowders; Wet-chemical synthesis; Reactive powder; Hydrogen peroxide
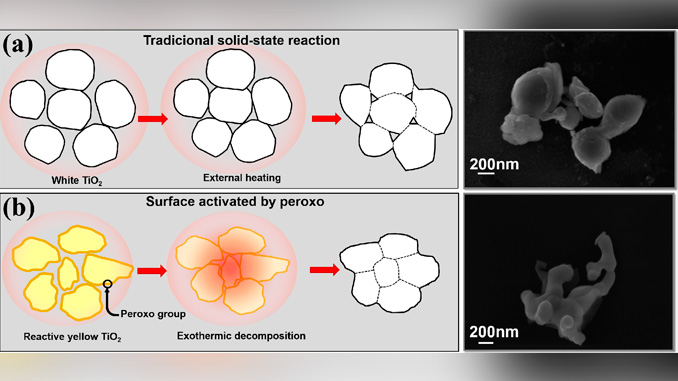
Abstract: Bismuth titanate with sillenite structure (Bi12TiO20) was prepared at lower temperatures and shorter times using a modified oxidant peroxide method (OPM). Bi12TiO20 was synthesized utilizing commercial Bi2O3 and reactive titanium dioxide nanoparticles having peroxo-modified surfaces. Rather than depending on particle size, the reaction mechanism is related to the highly exothermic decomposition of peroxo groups, regardless the titanium source used, which locally releases a large amount of energy that can accelerate the reaction, similar to self-propagating high temperature routes (SHS).