Oxidative dehydrogenation of ethylbenzene to styrene over the CoFe2O4-MCM-41 catalyst: preferential adsorption on the O2-Fe3+O2- sites located at octahedral positions
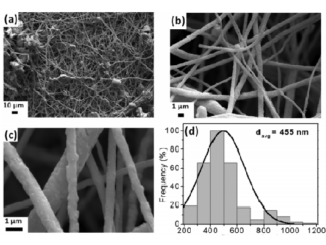
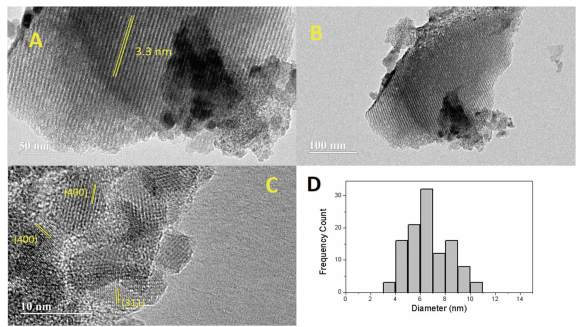
Abstract: The present study describes the catalytic performance of cobalt ferrite supported on MCM-41 for the oxidative dehydrogenation of ethylbenzene. The catalytic activity of cobalt ferrite was compared with that of the traditional hematite based catalyst. A mechanism is described indicating the role of the O2–Fe3+-O2- and O2–Co2+-O2- acid-base sites present in the tetrahedral and octahedral positions of the cobalt ferrite structure. The solids were characterized by X-ray diffraction (XRD), Raman spectroscopy (RS), Mossbauer spectroscopy (MS), X-ray photoelectron spectroscopy (XPS), vibrating-sample magnetometry (VSM), temperature-programmed reduction (H-2-TPR), chemical adsorption of NO and pyridine followed by infrared analysis, temperature programmed desorption of CO2 (TPD), N-2 physisorption and transmission electronic microscopy (TEM). The catalytic tests were performed in a fixed bed reactor using a saturator containing ethylbenzene. The XRD, RS, MS and VSM results confirmed the formation of cobalt ferrite, which was classified as partially inverted ferrite. The low-angle XRD, N-2 isotherms and TEM images show the formation of the mesoporous MCM-41 support with a high surface area. The catalytic tests confirmed that the cobalt ferrite is more active and stable than the traditional hematite catalyst. The catalytic cycle for ethylbenzene dehydrogenation occurs preferentially in the O2–Fe3+-O2- octahedral sites compared to the O2–Co2+-O2- sites. A theoretical approach using density functional theory revealed a higher acidity of iron sites compared to cobalt ones on the surface of the partially inverted spinel. The adsorption of ethylbenzene takes place preferentially in the outermost FeOx (x > 4) sites (Lewis acid) and the dehydrogenation reaction occurs predominantly in the oxygens bound to iron (Lewis base) according to the complementary electrostatic potential surface approach.
Author(s): Soares, MDB; Barbosa, FF; Torres, MAM; Valentini, A; Albuquerque, AD; Sambrano, JR; Pergher, SBC; Essayem, N; Braga, TP
CATALYSIS SCIENCE & TECHNOLOGY
Volume: 9 Pages: 2469-2484 Published: MAY 21 2019
DOI: 10.1039/c9cy00618d