Writers: R.C. Deus; M. Cilense; C.R. Foschini; M.A. Ramirez; E. Longo; A.Z. Simões
Keywords: Nanoparticle; Chemical synthesis; Microwave; Electron microscopy; Crystal structure
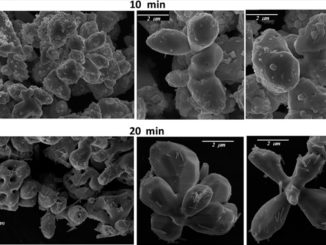
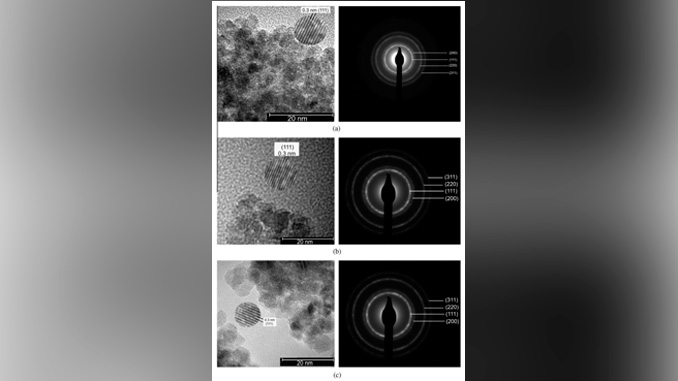
Abstract: Cystalline ceria (CeO2) nanoparticles have been synthesized by a simple and fast microwave-assisted hydrothermal (MAH) under NaOH, KOH, and NH4OH mineralizers added to a cerium ammonium nitrate aqueous solution. The products were characterized by X-ray powder diffraction (XRD), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), Fourier transformed-IR and Raman spectroscopies. Rietveld refinement reveals a cubic structure with a space group Fm3m while infrared data showed few traces of nitrates. Field emission scanning microcopy (FEG-SEM) revealed a homogeneous size distribution of nanometric CeO2 nanoparticles. The MAH process in KOH and NaOH showed most effective to dehydrate the adsorbed water and decrease the hydrogen bonding effect leaving a weakly agglomerated powder of hydrated ceria. TEM micrographs of CeO2 synthesized under MAH conditions reveal particles well-dispersed and homogeneously distributed. The MAH enabled cerium oxide to be synthesized at 100 °C for 8 min.