Writers: Wesley B. S. Machini, Marcos F. S. Teixeira
Keywords: Biomimetic oxo-manganese complex; Biomimetic activity; Electrocatalytic sulfite sensor
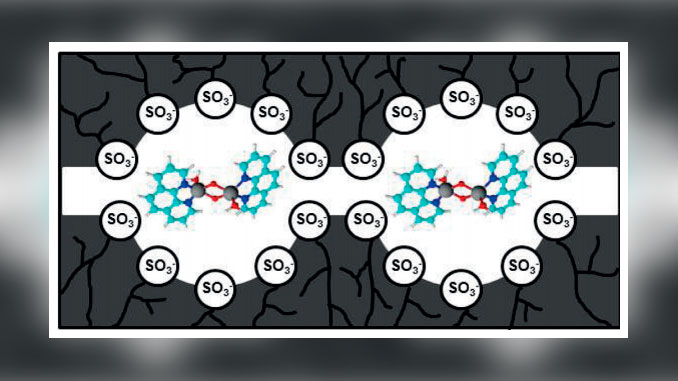
Abstract: A biomimetic sensor containing the oxobridged dinuclear manganese-phenanthroline complex incorporated into a cation-exchange polymeric film deposited onto glassy carbon electrode for detection of sulfite was studied. Cyclic voltammetry at the modified electrode in universal buffer showed a two electron oxidation/reduction of the couple MnIV(m-O)2MnIV/MnIII(mO)2MnIII. The sensor exhibited electrocatalytic property toward sulfite oxidation with a decrease of the overpotential of 450 mV compared with the glassy carbon electrode. A plot of the anodic current versus the sulfite concentration for potential fixed (+0.15 V vs. SCE) at the sensor was linear in the 4.99 107 to 2.49 106 molL1 concentration range and the concentration limit was 1.33 107 molL1 . The mediated mechanism was derived by MichaelisMenten kinetics. The calculated kinetics values were MichaelisMenten rate constant=Kapp M = 1.33 mmolL1 , catalytic rate constant=6.06 103 s 1 and heterogeneous electro-chemical rate constant=3.61 105 cm s1 .