Conformational study of the electronic interactions and nitric oxide release potential of new S-nitrosothiols esters derivatives of ibuprofen, naproxen and phenyl acids substituted (SNO-ESTERS): Synthesis, infrared spectroscopy analysis and theoretical calculations
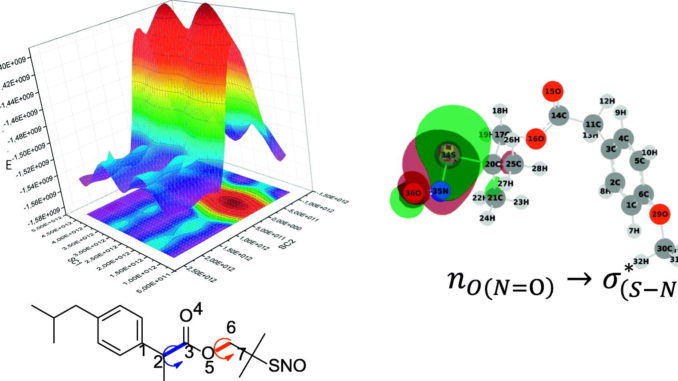
Abstract: The conformational study on the new S‑nitrosothiols esters (SNO-ESTERS): para–substituted (X = H, OMe, Cl and NO2) S‑nitrosothiol derivatives 2‑methyl‑2‑(sulfanyl)propyl phenylacetates (R1), 2‑(4‑isobutylphenyl)propanoate (ibuprofen, R2), and 2‑(4‑isobutylphenyl)propanoate of 2‑methyl‑2‑(nitrososulfanyl)propyl (naproxen, R3) was performed using infrared spectroscopy (IR) in solvents with increasing polarity (CCl4, CH3Cl, and CH3CN), and theoretical calculations, to determine the preferential conformer and the potential of these compounds to release nitric oxide (NO). S‑Nitrosothiols were synthesized by esterification reactions, using chlorides of the corresponding carboxylic acids, with good yields (~60%). IR results showed that these compounds presented only one conformation, and the experimental data were supported by the theoretical results obtained by density functional theory (DFT) calculations using the 6311+G (2df, 2p) basis set. The calculations revealed that all S‑nitrosothiols presented one preferential anticlinal (ac) geometric conformation, which agrees with the data obtained experimentally in CCl4. These conformers are stabilized by intramolecular hydrogen bonds. Examination of the geometry with regard to the RSNO group revealed that these compounds are preferentially in the trans (anti) conformation. The calculation of the orbital interactions using the Natural Bond Orbital (NBO) method showed that the nO(NO) → σ(S
N)∗ hyper-conjugative interaction increases the S
N bond length. The strong nS → π(NO)∗ interaction and electronic delocalization induces a partial πcharacter to the S
N bond. The weak σS
N bond indicates strong delocalization of the electron pair in O (NO) by the nO(NO) → σ(S
N)∗ interaction, thereby increasing the capacity of NO release from SNO-ESTERS.
Author(s): Reginato, M. M.; Paiva, D. R.; Sensato, F. R.; et al.
Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy
Volume: 207 Pages: 132-142 Published: 2019
DOI: https://doi.org/10.1016/j.saa.2018.09.020